Morphometric of ovaries, follicles, and their relationship with oocyte quality in cattle
DOI:
https://doi.org/10.15517/am.v34i1.50156Keywords:
oocyte quality, ovaries size, follicle sizeAbstract
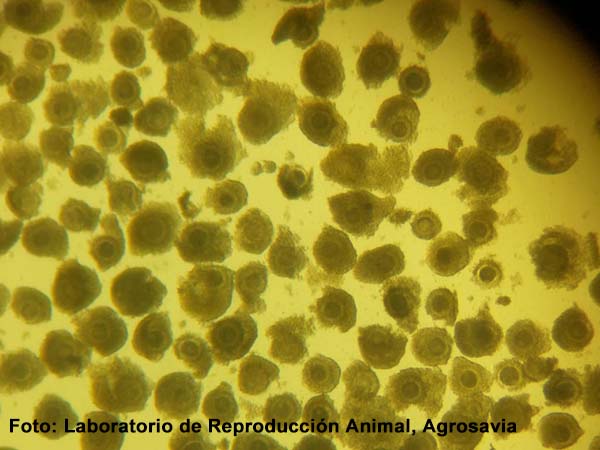
Introduction. Studies on the structural characteristics of the ovary associated with the quality of the cumulus-oocyte complex (COCs) in cattle contribute to the definition of strategies to improve reproductive efficiency. Objective. To determine the weight and size of the ovaries, follicle quality, and COCs in Zebu females, in order to establish differences between the right and left ovary. Materials and methods. Between March and July 2017, 52 ovaries were obtained from twenty-six Zebu cows. The ovaries and follicles were weighed and measured. The follicles were grouped into three categories: a) <3 mm (TFI), b) 3-6 mm (TFII) and c) >6 mm (TFIII). The follicles quality was determined by the irrigation-translucency technique (TIT) and the COCs by the characteristics of the cytoplasm and cumulus cells as good (CI), fair (CII), and poor (CIII), and by cresyl brilliant blue test were categorized as BCB+ and BCB-. Results. Ovarian length, width and weight were 2.7 ± 0.6 cm, 1.8 ± 0.5 cm, and 7.0 ± 3 g, respectively, with differences for length and weight between the right and left ovary (p<0.01). The highest number of follicles were obtained in TFI (p<0.05). The 88.56 % of the follicles evaluated were of good quality. A total of 52 % and 44.2 % of COCs were classified as CI and BCB+, respectively. When follicle size and COC quality were considered, it was shown that in the TFI group 22 % were IC, 34 % CII, and 44 % CIII, and in the TFII group 31 % were IC, 21 % CII and 48 % CIII. No differences were observed between left and right ovary. Conclusion. The right ovary presented a greater size and weight, a greater number of follicular structures and a greater number of good quality CCOs.
Downloads
References
Alba Gómez, L. O., Rodríguez Galindo, A., Gómez Palmero, A., & Silveira Prado, E. A. (2006). Tamaño y forma de los ovarios y del cérvix de hembras cebu de Cuba y sus relaciones con la eficiencia reproductiva. Revista Electrónica de Veterinaria, 7(3), 1–12.
Alm, H., Torner, H., Löhrke, B., Viergutz, T., Ghoneim, I. M., & Kanitz, W. (2005). Bovine blastocyst development rate in vitro is influenced by selection of oocytes by brillant cresyl blue staining before IVM as indicator for glucose-6-phosphate dehydrogenase activity. Theriogenology, 63(8), 2194–2205. https://doi.org/10.1016/j.theriogenology.2004.09.050
Argudo, D. E., Tenemaza, M. A., Merchán, S. L., Balvoa, J. A., Méndez, M. S., Soria, M. E., Galarza, L. R., Ayala, L. E., Hernández-Fonseca, H. J., Perea, M. S., & Perea, F. P. (2020). Intraovarian influence of bovine corpus luteum on oocyte morphometry and developmental competence, embryo production and cryotolerance. Theriogenology, 155, 232–239. https://doi.org/10.1016/j.theriogenology.2020.05.044
Bhojwani, S., Alm, H., Torner, H., Kanitz, W., & Poehland, R. (2007). Selection of developmentally competent oocytes through brilliant cresyl blue stain enhances blastocyst development rate after bovine nuclear transfer. Theriogenology, 67(2), 341–345. https://doi.org/10.1016/j.theriogenology.2006.08.006
Castaneda, C. A., Kaye, P., Pantaleon, M., Phillips, N., Norman, S., Fry, R., & D’Occhio, M. J. (2013). Lipid content, active mitochondria and brilliant cresyl blue staining in bovine oocytes. Theriogenology, 79(3), 417–422. https://doi.org/10.1016/j.theriogenology.2012.10.006
Chaubal, S. A., Molina, J. A., Ohlrichs, C. L., Ferre, L. B., Faber, D. C., Bols, P. E. J., Riesen, J. W., Tian, X., & Yang, X. (2006). Comparison of different transvaginal ovum pick-up protocols to optimise oocyte retrieval and embryo production over a 10-week period in cows. Theriogenology, 65(8), 1631–1648. https://doi.org/10.1016/j.theriogenology.2005.07.020
de Loos, F., van Vliet, C., van Maurik, P., & Kruip, T. A. M. (1989). Morphology of immature bovine oocytes. Gamete Research, 24(2), 197–204. https://doi.org/10.1002/mrd.1120240207
Dorice, A. K., Ferdinand, N., Justin, K., Augustave, K., & Linda, K. K. (2019). Effects of breed, age, body condition score, and nutritional status on follicular population, oocyte yield, and quality in three Cameroonian zebus cattle Bos indicus. Advances in Agriculture, 2019, Article 2979740. https://doi.org/10.1155/2019/2979740
Dyce, K. M., Sack, W. O., & Wensing, C. J. G. (1991). Anatomía veterinaria. Médica Panamericana.
Estrella, C., Suconota, G., & Ayala, L. (2017). Evaluación de la calidad de ovocitos bovinos obtenidos de folículos con tres tamaños diferentes. Maskana, 8, 101–103. https://publicaciones.ucuenca.edu.ec/ojs/index.php/maskana/article/view/1499
Filipiak, Y., Viqueira, M., & Bielli, A. (2016). Desarrollo y dinámica de los folículos ováricos desde la etapa fetal hasta la prepuberal en bovinos. Veterinaria (Montevideo), 52(202), 14–22. https://www.produccion-animal.com.ar/informacion_tecnica/inseminacion_artificial/253-Bielli.pdf
Fukuda, M., Fukuda, K., Andersen, C. Y., & Byskov, A. G. (2000). Right-sided ovulation favours pregnancy more than left-sided ovulation. Human Reproduction, 15(9), 1921–1926. https://doi.org/10.1093/humrep/15.9.1921
Gandolfi, F., Brevini, T. A. L., Cillo, F., & Antonini, S. (2005). Cellular and molecular mechanisms regulating oocyte quality and the relevance for farm animal reproductive efficiency. Revue Scientifique et Technique, 24(1), 413–423. https://doi.org/10.20506/rst.24.1.1580
Gereš, D., Ževrnja, B., Žubčić, D., Zobel, R., Vulić, B., Staklarević, N., & Gracin, K. (2011). Asymmetrical functional activities of ovaries and tubular part of reproductive organs of dairy cows. Veterinarski Arhiv, 81(2), 187–198. https://hrcak.srce.hr/file/100549
Ginther, O. J. (2019). Intraovarian spatial and vascular harmony between follicles and corpus luteum in monovulatory heifers, mares, and women. Theriogenology, 128, 31–39. https://doi.org/10.1016/j.theriogenology.2019.01.019
Ginther, O. J., & Dangudubiyyam, S. V. (2019). Role of intraovarian mechanisms and side of ovary on characteristics of follicle selection in Bos taurus heifers. Theriogenology, 135, 56–64. https://doi.org/10.1016/j.theriogenology.2019.05.027
Ginther, O. J., & Gomez-León, V. E. (2020). Intraovarianism and differences in ovulation frequency between left and right ovaries in Bos taurus heifers. Reproductive Biology, 20(3), 333–337. https://doi.org/10.1016/j.repbio.2020.05.006
Ginther, O. J., & Hoffman, M. M. (2014). Intraovarian effect of dominant follicle and corpus luteum on number of follicles during a follicular wave in heifers. Theriogenology, 82(1), 169–175. https://doi.org/10.1016/j.theriogenology.2014.03.013
González Tous, M., Pastrana Puche, N., Barón Pinedo, F., & Vertel Morrison, M. (2014). Frecuencia de presentación de gestación con relación al cuerno uterino en bovinos del trópico colombiano. Revista de Medicina Veterinaria, 28, 13–21.
Karami-Shabankareh, H., & Mirshamsi, S. M. (2012). Selection of developmentally competent sheep oocytes using the brilliant cresyl blue test and the relationship to follicle size and oocyte diameter. Small Ruminant Research, 105(1–3), 244–249. https://doi.org/10.1016/j.smallrumres.2012.02.017
Kruip, T. A. M., & Dieleman, S. J. (1982). Macroscopic classification of bovine follicles and its validation by micromorphological and steroid biochemical procedures. Reproduction Nutrition Développement, 22(3), 465–473. https://doi.org/10.1051/rnd:19820403
Lamas-Toranzo, I., Pericuesta, E., & Bermejo-Álvarez, P. (2018). Mitochondrial and metabolic adjustments during the final phase of follicular development prior to IVM of bovine oocytes. Theriogenology, 119, 156–162. https://doi.org/10.1016/j.theriogenology.2018.07.007
Leal, L. S., Moya-Araújo, C. F., Fernandes, C. B., Martins, L. R., Landim-Alvarenga F.C, & Oba, E. (2010). Evaluation of recovery, quality and in vitro nuclear maturation of oocytes obtained from Buffalo and Bovine Ovaries. Revista Veterinaria, 21(Supplement 1), 892–894.
Leibfried, L., & First, N. L. (1979). Characterization of bovine follicular oocytes and their ability to mature in vitro. Journal of Animal Science, 48(1), 76–86. https://doi.org/10.2527/jas1979.48176x
Lequarre, A. -S., Vigneron, C., Ribaucour, F., Holm, P., Donnay, I., Dalbiès-Tran, R., Callesen, H., & Mermillod, P. (2005). Influence of antral follicle size on oocyte characteristics and embryo development in the bovine. Theriogenology, 63(3), 841–859. https://doi.org/10.1016/j.theriogenology.2004.05.015
Lonergan, P., Gutiérrez-Adán, A., Rizos, D., Pintado, B., de La Fuente, J., & Boland, M. P. (2003). Relative messenger RNA abundance in bovine oocytes collected in vitro or in vivo before and 20 hr after the preovulatory luteinizing hormone surge. Molecular Reproduction and Development, 66(3), 297–305. https://doi.org/10.1002/mrd.10357
Lonergan, P., Monaghan, P., Rizos, D., Boland, M. P., & Gordon, I. (1994). Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Molecular Reproduction and Development, 37(1), 48–53. https://doi.org/10.1002/mrd.1080370107
Machatkova, M., Krausova, K., Jokesova, E., & Tomanek, M. (2004). Developmental competence of bovine oocytes: effects of follicle size and the phase of follicular wave on in vitro embryo production. Theriogenology, 61(2–3), 329–335. https://doi.org/10.1016/S0093-691X(03)00216-4
Mirshamsi, S. M., KaramiShabankareh, H., Ahmadi-Hamedani, M., Soltani, L., Hajarian, H., & Abdolmohammadi, A. R. (2013). Combination of oocyte and zygote selection by brilliant cresyl blue (BCB) test enhanced prediction of developmental potential to the blastocyst in cattle. Animal Reproduction Science, 136(4), 245–251. https://doi.org/10.1016/j.anireprosci.2012.11.002
Moreira Viana, J. H., de Almeida Camargo, L. S., de Moraes Ferreira, A., Ferreira de Sa, W., de Carvalho Fernandes, C. A., & de Pinho Marques Junior, A. (2004). Short intervals between ultrasonographically guided follicle aspiration improve oocyte quality but do not prevent establishment of dominant follicles in the Gir breed (Bos indicus) of cattle. Animal Reproduction Science, 84(1–2), 1–12. https://doi.org/10.1016/j.anireprosci.2003.12.002
Neves, M. M., Marques Jr., A. P., Santana, C. V., Lima, F. P. C., & Zambrano, W. J. (2002). Características de ovários de fêmeas zebu (Bos taurus indicus), colhidos em abatedouros. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 54(6), 651–654. https://doi.org/10.1590/S0102-09352002000600016
Nunes-Dode, M. A., Rodovalho, N. C., Ueno, V. G., & Alves, R. G. O. (2001). Oocyte recovering from Bos indicus females. Arch Zootec., 50, 415–418.
Opiela, J., & Kątska-Książkiewicz, L. (2013). The utility of Brilliant Cresyl Blue (BCB) staining of mammalian oocytes used for in vitro embryo production (IVP). Reproductive Biology, 13(3), 177–183. https://doi.org/10.1016/j.repbio.2013.07.004
Opiela, J., Kątska-Książkiewicz, L., Lipiński, D., Słomski, R., Bzowska, M., & Ryńska, B. (2008). Interactions among activity of glucose-6-phosphate dehydrogenase in immature oocytes, expression of apoptosis-related genes Bcl-2 and Bax, and developmental competence following IVP in cattle. Theriogenology, 69(5), 546–555. https://doi.org/10.1016/j.theriogenology.2007.11.001
Pavlok, A., Lucas-Hahn, A., & Niemann, H. (1992). Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Molecular Reproduction and Development, 31(1), 63–67. https://doi.org/10.1002/mrd.1080310111
Pontes, J. H. F., Melo Sterza, F. A., Basso, A. C., Ferreira, C. R., Sanches, B. V., Rubin, K. C. P., & Seneda, M. M. (2011). Ovum pick up, in vitro embryo production, and pregnancy rates from a large-scale commercial program using Nelore cattle (Bos indicus) donors. Theriogenology, 75(9), 1640–1646. https://doi.org/10.1016/j.theriogenology.2010.12.026
Pujol, M., López-Béjar, M., & Paramio, M. -T. (2004). Developmental competence of heifer oocytes selected using the brilliant cresyl blue (BCB) test. Theriogenology, 61(4), 735–744. https://doi.org/10.1016/S0093-691X(03)00250-4
Quintana, M. D., Campos, P. E. C., Herrera, P., Gallego, C., & Padrón, E. (2012). Comparación de dos métodos de recolección de ovocitos inmaduros para fertilización in vitro FIV obtenidos de hembras Bubalus bubalis enviadas a matadero. Revista de Salud Animal, 34(1), 53–56. http://revistas.censa.edu.cu/index.php/RSA/article/view/73
Rizos, D., Burke, L., Duffy, P., Wade, M., Mee, J. F., O’Farrell, K. J., MacSiurtain, M., Boland, M. P., & Lonergan, P. (2005). Comparisons between nulliparous heifers and cows as oocyte donors for embryo production in vitro. Theriogenology, 63(3), 939–949. https://doi.org/10.1016/j.theriogenology.2004.05.008
Rodríguez, E., López, M., Izquierdo, D., & Paramio, M. (2003). Developmental competence of prepubertal goat oocytes selected with brilliant cresyl blue and matured with cysteamine supplementation. Reproduction Nutrition Development, 43(2), 179–87. https://doi.org/10.1051/rnd:2003012
Saini, N., Singh, M. K., Shah, S. M., Singh, K. P., Kaushik, R., Manik, R. S., Singla, S. K., Palta, P., & Chauhan, M. S. (2015). Developmental competence of different quality bovine oocytes retrieved through ovum pick-up following in vitro maturation and fertilization. Animal, 9(12), 1979–1985. https://doi.org/10.1017/S1751731115001226
Sánchez, A. (2007). Morfometría ovárica de hembras Cebú (Bos Indicus). Cultura Científica, 5, 61–64. https://revista.jdc.edu.co/index.php/Cult_cient/article/view/324
Siqueira Caixeta, E., & Alves Nunes Dode, M. (2008). Dissecação folicular: um método eficiente para estudos de competência ovocitária. Embrapa Genetic Resources & Biotechnology. https://bit.ly/3MfnGf4
Souza-Cácares, M. B., Fialho, A. L. L., Silva, W. A. L., Cardoso, C. J. T., Pöhland, R., Martins, M. I. M., & Melo-Sterza, F. A. (2019). Oocyte quality and heat shock proteins in oocytes from bovine breeds adapted to the tropics under different conditions of environmental thermal stress. Theriogenology, 130, 103–110. https://doi.org/10.1016/j.theriogenology.2019.02.039
Stevenson, J. S. (2019). Spatial relationships of ovarian follicles and luteal structures in dairy cows subjected to ovulation synchronization: Progesterone and risks for luteolysis, ovulation, and pregnancy. Journal of Dairy Science, 102(6), 5686–5698. https://doi.org/10.3168/jds.2018-16036
Tan, S. J., & Lu, K. H. (1990). Effects of different oestrous stages of ovaries and sizes of follicles on generation of bovine embryos in vitro. Theriogenology, 33(1), 335. https://doi.org/10.1016/0093-691X(90)90759-M
Torner, H., Ghanem, N., Ambros, C., Hölker, M., Tomek, W., Phatsara, C., Alm, H., Sirard, M.-A., Kanitz, W., Schellander, K., & Tesfaye, D. (2008). Molecular and subcellular characterisation of oocytes screened for their developmental competence based on glucose-6-phosphate dehydrogenase activity. Reproduction, 135(2), 197–212. https://doi.org/10.1530/REP-07-0348
Viana, J. H. M., Palhao, M. P., Siqueira, L. G. B., Fonseca, J. F., & Camargo, L. S. A. (2010). Ovarian follicular dynamics, follicle deviation, and oocyte yield in Gyr breed (Bos indicus) cows undergoing repeated ovum pick-up. Theriogenology, 73(7), 966–972. https://doi.org/10.1016/j.theriogenology.2009.11.025
Wurth, Y. A., Boni, R., Hulshof, S. C. J., & Kruip, T. A. M. (1994). Bovine embryo production in vitro after selection of ovaries, follicles and oocytes. Utrecht University Press.
Yamamoto, T., Iwata, H., Goto, H., Shiratuki, S., Tanaka, H., Monji, Y., & Kuwayama, T. (2010). Effect of maternal age on the developmental competence and progression of nuclear maturation in bovine oocytes. Molecular Reproduction and Development, 77(7), 595–604. https://doi.org/10.1002/mrd.21188
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 ELIANA NEIRA RIVERA

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
1. Proposed policy for open access journals
Authors who publish in this journal accept the following conditions:
a. Authors retain the copyright and assign to the journal the right to the first publication, with the work registered under the attribution, non-commercial and no-derivative license from Creative Commons, which allows third parties to use what has been published as long as they mention the authorship of the work and upon first publication in this journal, the work may not be used for commercial purposes and the publications may not be used to remix, transform or create another work.
b. Authors may enter into additional independent contractual arrangements for the non-exclusive distribution of the version of the article published in this journal (e.g., including it in an institutional repository or publishing it in a book) provided that they clearly indicate that the work was first published in this journal.
c. Authors are permitted and encouraged to publish their work on the Internet (e.g. on institutional or personal pages) before and during the review and publication process, as it may lead to productive exchanges and faster and wider dissemination of published work (see The Effect of Open Access).