Fast seed histology protocols: Benzene derivatives-free vs xylene-dependent
DOI:
https://doi.org/10.15517/am.v33iEspecial.51308Keywords:
periodic acid of Schiff, protein staining, microwave processing, Coomassie blueAbstract
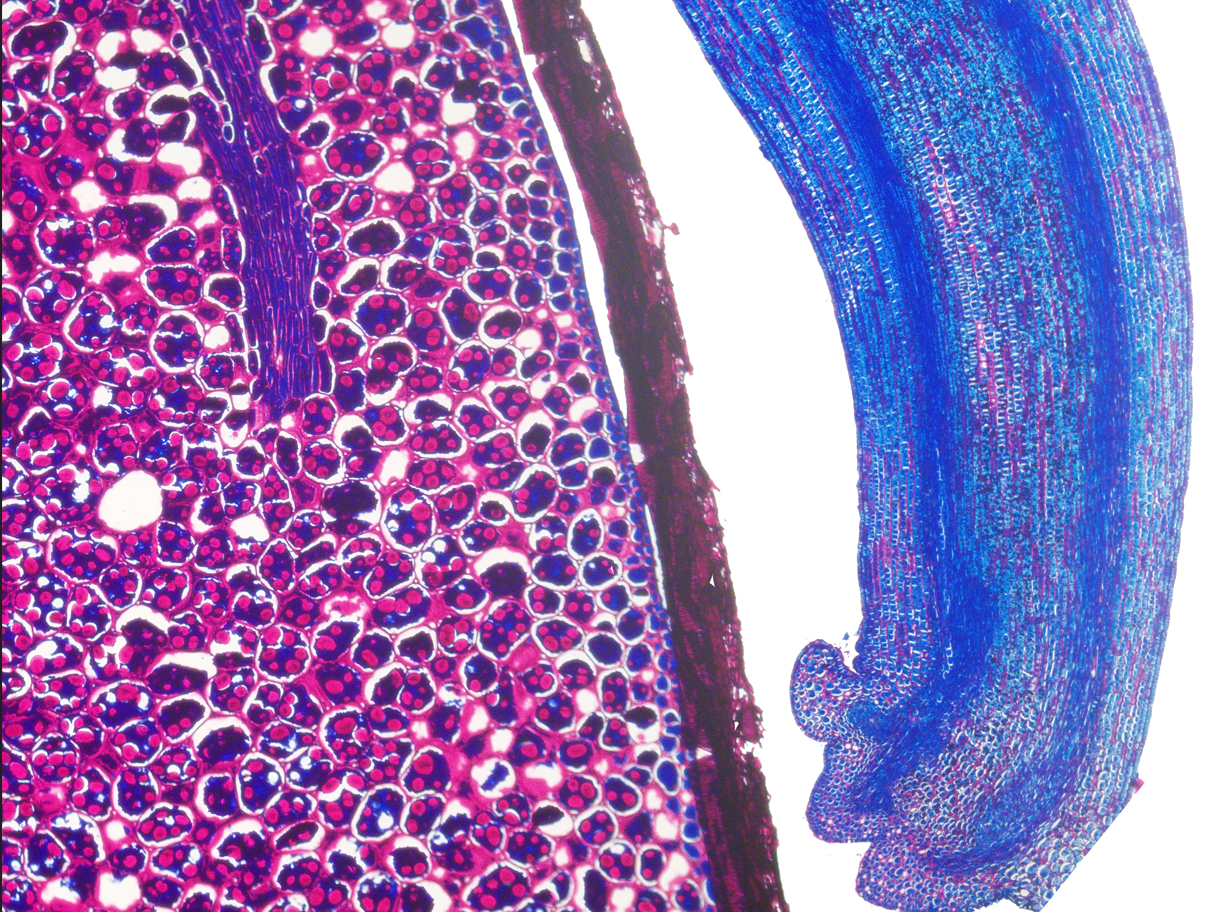
Introduction. Seeds are complex structures that allow the biological and crop propagation of plants. Seed histology can be used for teaching, researching, and for pathological diagnostic. Histology protocols are commonly divided into 5 different stages: fixation, processing, cutting, staining, and mounting. Xylene is a dangerous reagent used during the processing, staining, and mounting of histological specimens that can contaminate the environment and is toxic for users. Objective. To compare two new protocols for seed histology accelerated with microwave, tested on seeds of economic importance species. Materials and methods. The experiments were done between January and May of 2022 at the Centro de Investigaciones en Granos y Semillas (CIGRAS) of the Universidad de Costa Rica. The compared protocols were: a benzene derivatives-free (BDF) and a xylene-dependent (XD). Seeds of Carica papaya L. (Caricaceae) var. Pococí, Coffea arabica L. (Rubiaceae) var. Obata, Glycine max L. (Fabaceae) var. CIGRAS-06, Phaseolus vulgaris L. (Fabaceae) var. Tayni, Oryza sativa L. (Poaceae) var. Lazarroz FL, and Zea mays L. (Poaceae) var. EJN-2 were used. Three technical replicates of five seeds of each species were tested with the XD and BDF protocols, on different days each replicate. Results. The photomicrographs obtained with both protocols showed that the samples maintained the morphology integrity of embryo, endosperm or cotyledons, and other seed structures. BDF and XD protocols produced seed histology slides and microphotographs. PAS-Coomassie Blue staining made a good differentiation of carbohydrates and proteins. Fastness of both protocols is a benefit compared with other protocols for plant histology that can take several days or even weeks. Conclusions. The BDF and XD protocols were suitable for seed histology analysis of bean, coffee, maize, papaya, and soybean, slides were obtained in less than 5 hours. BDF protocol is the first for plant tissue processing that does not use benzene derivatives and that uses paraffin as embedding medium.
Downloads
References
Alamri, A., Yeb, J., & Blancato, J. (2017). Fluorescence in situ hybridization of cells, chromosomes, and formalin-fixed paraffin-embedded tissues. In J. M. Walker (Ed.), Methods in molecular biology (pp. 265–279). Humana Press. https://doi.org/10.1007/978-1-4939-6990-6
Alturkistani, H. A., Tashkandi, F. M., & Mohammedsaleh, Z. M. (2015). Histological stains: A literature review and case study. Global Journal of Health Science, 8(3), 72–79. https://doi.org/10.5539/gjhs.v8n3p72
Amaral da Silva, E. A., Toorop, P. E., van Aelst, A. C., & Hilhorst, H. W. M. (2004). Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta, 220, 251–261. https://doi.org/10.1007/s00425-004-1344-0
Amaral da Silva, E. A., Toorop, P. E., van Lammeren, A. A. M., & Hilhorst, H. W. M. (2008). ABA inhibits embryo cell expansion and early cell division events during coffee (Coffea arabica ’Rubi’) seed germination. Annals of Botany, 102(3), 425–433. https://doi.org/10.1093/aob/mcn112
Araujo Oliveira, L., de Souza, G. A., Tavares Silva, B., Gomes Rocha, A. A., de Toledo Picoli, E. A., de Souza Pereira, D., Lopes Donzeles, S. M., de Freitas Ribeiro, M., & Marques Ferreira, W. P. (2020). Histochemical approach of the mobilization of reserve compounds in germinating coffee seeds. Coffee Science, 15(1), 1–14. https://doi.org/10.25186/.v15i.1704
Ayotamuno, M. J., Kogbara, R. B., Ogaji, S. O. T., & Probert, S. D. (2006). Petroleum contaminated ground-water: Remediation using activated carbon. Applied Energy, 83(11), 1258–1264. https://doi.org/10.1016/j.apenergy.2006.01.004
Becker, K., Jährling, N., Saghafi, S., Weiler, R., & Dodt, H. U. (2012). Chemical clearing and dehydration of GFP expressing mouse brains. PLoS ONE, 7(3), Article e33916. https://doi.org/10.1371/journal.pone.0033916
Benavides-Acevedo, M. (2021). Caracterización genética, genómica e histológica de una mutación sexual en Carica papaya (CARICACEAE) [Tesis de Maestría, Universidad de Costa Rica]. Repositorio Kérwá. https://www.kerwa.ucr.ac.cr/handle/10669/85284
Bracegirdle, B. (1977). The history of histology: A brief survey of sources. History of Science, 15(2), 77–101. https://doi.org/10.1177/007327537701500201
Bradbury, S., & Meek, G. A. (1960). A study of potassium permanganate “fixation” for electron microscopy. Quarterly Journal of Microscopical Science, 101(3), 241–250. https://doi.org/10.1242/jcs.s3-101.55.241
Buesa, R. J. (2007a). Histology safety: Now and then. Annals of Diagnostic Pathology, 11(5), 334–339. https://doi.org/10.1016/j.anndiagpath.2007.06.005
Buesa, R. J. (2007b). Microwave-assisted tissue processing: Real impact on the histology workflow. Annals of Diagnostic Pathology, 11(3), 206–211. https://doi.org/10.1016/j.anndiagpath.2007.02.006
Buesa, R. J., & Peshkov, M. V. (2009). Histology without xylene. Annals of Diagnostic Pathology, 13(4), 246–256. https://doi.org/10.1016/j.anndiagpath.2008.12.005
Buides, J. A. F., Álvarez, A. G., La Fé, P. L. C., & Sánchez, G. G. (2017). Aspectos anatómicos y viabilidad de semillas de papaya (Carica papaya L.) variedad “Maradol Roja” sometidas a almacenamento prolongado. Agrotecnia de Cuba, 41(1), 41–51.
Dedecca, D. M. (1957). Anatomia e desenvolvimento ontogenético de Coffea arabica L. var. typica Cramer. Bragantia, 16, 315–366. https://doi.org/10.1590/s0006-87051957000100023
Duan, W., Meng, F., Wang, F., & Liu, Q. (2017). Environmental behavior and eco-toxicity of xylene in aquatic environments: A review. Ecotoxicology and Environmental Safety, 145, 324–332. https://doi.org/10.1016/j.ecoenv.2017.07.050
Eltoum, I., Fredenburgh, J., Myers, R. B., & Grizzle, W. E. (2001). Introduction to the theory and practice of fixation of tissues. Journal of Histotechnology, 24(3), 173–190. https://doi.org/10.1179/his.2001.24.3.173
Everson-Pearse, A. (1954). Histochemistry: theoretical and applied. Journal of Chemical Education, 31(7), 397. https://doi.org/10.1021/ed031p391.1
Feder, N., & O’Brien, T. P. (1968). Plant Microtechnique: Some Principles and New Methods. American Journal of Botany, 55(1), 123–142. https://doi.org/10.1002/j.1537-2197.1968.tb06952.x
Fernandes, F. A. N., Gallão, M. I., & Rodrigues, S. (2009). Effect of osmosis and ultrasound on pineapple cell tissue structure during dehydration. Journal of Food Engineering, 90(2), 186–190. https://doi.org/10.1016/j.jfoodeng.2008.06.021
Fhaizal, M., Bukhori, M., Jin, S., Pillai, V., & Rahman, N. A. (2013). Improved protocol for high frequency plant regeneration through somatic embryogenesis in Carica papaya. Cultures, 4(5), 9–19. https://updatepublishing.com/journal/index.php/rib/article/view/2441
Forti, V. A., de Carvalho, C., André, F., Tanaka, O., & Cicero, S. M. (2013). Weathering damage in soybean seeds: Assessment, seed anatomy and seed physiological potential. Journal of Seed Technology, 35(2), 213–224. http://www.jstor.org/stable/24642271
Fresneda, J. A., González, A. A., González, P. L., & Guibert, G. (2017). Aspectos anatómicos y viabilidad de semillas de papaya (Carica papaya L.) variedad “Maradol roja” sometidas a almacenamiento prolongado. Agrotecnia de Cuba, 41(1), 45-46. https://www.grupoagricoladecuba.gag.cu/media/Agrotecnia/pdf/2017/1/5.pdf
García, R. (1993). Laboratorio de anatomía patológica. Interamericana McGraw Hill.
Gil, A. I., & Miranda, D. (2011). Aspectos anatómicos de la semilla de papaya (Carica papaya L.). Revista Colombiana de Ciencias Hortícolas, 2(2), 145–156. https://doi.org/10.17584/rcch.2008v2i2.1183
Harris, H. C., & Brolmann, J. B. (1966). Comparison of calcium and boron deficiencies of the peanut II. Seed quality in relation to histology and viability. Agronomy Journal, 58(1), 578–583. https://doi.org/10.2134/agronj1966.00021962005800060008x
Kaplan, A., Çölgeçen, H., & Büyükkartal, N. (2009). Seed morphology and histology of some paronycia taxa (Caryophyllaceae) from Turkey. Bangladesh Journal of Botany, 38(2), 171–176.
Kelly, K. M., Van Staden, J., & Bell, W. E. (1992). Seed coat structure and dormancy. Plant Growth Regulation, 11(3), 201–209. https://doi.org/10.1007/BF00024559
Komarnytsky, S., Retchin, S., Vong, C. I., & Lila, M. A. (2022). Gains and losses of agricultural food production: Implications for the Twenty-First Century. Annual Review of Food Science and Technology, 13(1), 239–261. https://doi.org/10.1146/annurev-food-082421-114831
Kshatriya, K., Whitehill, J. G. A., Madilao, L., Henderson, H., Kermode, A., Kolotelo, D., & Bohlmann, J. (2018). Histology of resin vesicles and oleoresin terpene composition of conifer seeds. Canadian Journal of Forest Research, 48(9), 1073–1084. https://doi.org/10.1139/cjfr-2018-0164
Leong, A. S. -Y. (2004). Microwaves and turnaround times in histoprocessing: Is this a new era in histotechnology? American Journal of Clinical Pathology, 121(4), 460–462. https://doi.org/10.1309/PLQ523DENNH8R00Q
Liu, W. Y., Chang, Y. M., Chen, S. C. C., Lu, C. H., Wu, Y. H., Lu, M. Y., Chen, D. R., Shih, A. C. C., Sheue, C. R., Huang, H. C., Yu, C. P., Lin, H. H., Shiu, S. H., Ku, M. S. B., & Li, W. H. (2013). Anatomical and transcriptional dynamics of maize embryonic leaves during seed germination. Proceedings of the National Academy of Sciences of the United States of America, 110(10), 3979–3984. https://doi.org/10.1073/pnas.1301009110
Martin, C., & Sofla, A. Y. N. (2010). A method for bonding PDMS without using plasma. ASME International Mechanical Engineering Congress and Exposition, Proceedings, 10, 557–560. https://doi.org/10.1115/IMECE2010-38790
Materials and Safety Committee. (2015). Safety data sheet (SDS) Xylene. PCS. https://www.pcs.com.sg/wp-content/uploads/2017/04/PCS08006.pdf
McMillan, D. B., & Harris, R. J. (2018). Introduction. In D. B. McMillan, & R. J. Harris (Eds.), An atlas of comparative vertebrate histology (pp. ix–xxix). Academic Press. https://doi.org/10.1016/b978-0-12-410424-2.00018-4
Mendes de Jesus, V. A., Araújo, E. F., Santos, F. L., Alves, E., & dos Santos Dias, L. A. (2015). Sodium hypochlorite for sarcotesta remotion from papaya seeds: Anatomical studies. Journal of Seed Science, 37(4), 228–235. https://doi.org/10.1590/2317-1545v37n4153890
Mishra, S., & Vijayakumar, M. (2014). Phytochemical analysis and histology of Strychnos potatorum l. seeds. Journal for Drugs and Medicines, 6(2), 17–24. https://doi.org/10.15254/H.J.D.MED.6.2014.132
Mochizuki, Y., & Furukawa, K. (1987). Application of Coomassie blue staining to cultured hepatocytes. Cell Biology International Reports, 11(5), 367–371. https://bit.ly/3yhVx2T
Munganyinka, E., Margaria, P., Sheat, S., Ateka, E. M., Tairo, F., Ndunguru, J., & Winter, S. (2018). Localization of cassava brown streak virus in Nicotiana rustica and cassava Manihot esculenta (Crantz) using RNAscope® In Situ hybridization. Virology Journal, 15, Article 128. https://doi.org/10.1186/s12985-018-1038-z
Neuparth, T., Capela, R., Pereira, S. P. P., Moreira, S. M., Santos, M. M., & Reis-Henriques, M. A. (2014). Toxicity effects of hazardous and noxious substances (hns) to marine organisms: Acute and chronic toxicity of p -xylene to the amphipod Gammarus locusta. Journal of Toxicology and Environmental Health - Part A: Current Issues, 77(20), 1210–1221. https://doi.org/10.1080/15287394.2014.921867
Niaz, K., Bahadar, H., Maqbool, F., & Abdollahi, M. (2015). A review of environmental and occupational exposure to xylene and its health concerns. EXCLI Journal, 14, 1167–1186. https://doi.org/10.17179/excli2015-623
Ossai, I. C., Ahmed, A., Hassan, A., & Hamid, F. S. (2020). Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environmental Technology and Innovation, 17, Article 100526. https://doi.org/10.1016/j.eti.2019.100526
Paredes, M., Becerra, V., Donoso, G., Olmos, S., Rodríguez, R. (2021). Morfología y estados de crecimiento y desarrollo de la planta de arroz. En M. Paredes, V. Becerra, & G. Donoso (Eds.), 100 años del cultivo del arroz en Chile en un contexto internacional 1920-2020 (Tomo II; Cap. 14; pp. 408–445). Instituto de Investigaciones Agropecuarias. https://hdl.handle.net/20.500.14001/68052
Prophet, E., Mills, B., Arrington, J., & Sobin, L. (Eds.). (1995). Métodos histotecnológicos. Instituto de Patología de las Fuerzas Armadas de los Estados Unidos de América, Registro de Patología de los Estados Unidos de América, & Instituto de Patología de las Fuerzas Armadas de los Estados Unidos de América.
Rajan, S. T., & Malathi, N. (2014). Health hazards of xylene: A literature review. Journal of Clinical and Diagnostic Research, 8(2), 271–274. https://doi.org/10.7860/JCDR/2014/7544.4079
Rajjou, L., Duval, M., Gallardo, K., Catusse, J., Bally, J., Job, C., & Job, D. (2012). Seed germination and vigor. Annual Review of Plant Biology, 63, 507–533. https://doi.org/10.1146/annurev-arplant-042811-105550
Ramanadane, T., & Ponnuswamy, A. S. (2004). Ageing and anatomical influence on seed storability in rice (Oryza sativa L.) hybrids and parental lines. Tropical Agricultural Research, 16, 37–50.
Recek, N., Holc, M., Vesel, A., Zaplotnik, R., Gselman, P., Mozetiˇc, M., Primc, G. (2021). Germination of Phaseolus vulgaris L. sedes after a short treatment with a powerful RF plasma. International Journal of Molecular Sciences, 22(13), Article 6672. https://doi.org/10.3390/ijms22136672
Rogers, H. S., Donoso, I., Traveset, A., & Fricke, E. C. (2021). Cascading impacts of seed disperser loss on plant communities and ecosystems. Annual Review of Ecology, Evolution, and Systematics, 52, 641–666. https://doi.org/10.1146/annurev-ecolsys-012221-111742
Rolls, G., Farmer, N., & Hall, J. (2021). Artefactos en preparaciones histológicas y citológicas. Leica Biosystems. https://bit.ly/3Cc1LUd
Rousseau, D., Widiez, T., Tommaso, S., Rositi, H., Adrien, J., Maire, E., Langer, M., Olivier, C., Peyrin, F., & Rogowsky, P. (2015). Fast virtual histology using X-ray in-line phase tomography: Application to the 3D anatomy of maize developing seeds. Plant Methods, 11, Article 55. https://doi.org/10.1186/s13007-015-0098-y
Roy, D. (1999). Histology and pathology laboratories. Chemical hazard prevention and medical/health surveillance. American Association of Occupational Health Nurses Journal, 47(5), 199–205. https://journals.sagepub.com/doi/pdf/10.1177/216507999904700502
Rylander, C. G., Stumpp, O. F., Milner, T. E., Kemp, N. J., Mendenhall, J. M., Diller, K. R., & Welch, A. J. (2006). Dehydration mechanism of optical clearing in tissue. Journal of Biomedical Optics, 11(4), Article 041117. https://doi.org/10.1117/1.2343208
Sagara, T., Bhandari, D. R., Spengler, B., & Vollmann, J. (2020). Spermidine and other functional phytochemicals in soybean seeds: Spatial distribution as visualized by mass spectrometry imaging. Food Science and Nutrition, 8(1), 675–682. https://doi.org/10.1002/fsn3.1356
Samavedi, S., Poindexter, L. K., Van Dyke, M., & Goldstein, A. S. (2014). Chapter 7. Synthetic biomaterials for regenerative medicine applications. In G. Orlando, J. Lerut, S. Soker, & R. J. Stratta (Eds.), Regenerative medicine applications in organ transplantation (pp. 81–99). Elsevier Inc. https://doi.org/10.1016/B978-0-12-398523-1.00007-0
Sandoval, E. (2005). Técnicas aplicadas al estudio de la anatomía vegetal. Universidad Nacional Autónoma de México.
Santos de Oliveira, J. M. (2015). Simultaneous dehydration and infiltration with (2-hydroxyethyl)- methacrylate (HEMA) for lipid preservation in plant tissues. Acta Botanica Brasilica, 29(2), 207–212. https://doi.org/10.1590/0102-33062014abb3755
Schmidt, É. C., Pereira, B., Pontes, C. L. M., dos Santos, R., Scherner, F., Horta, P. A., de Martins, R. P., Latini, A., Maraschin, M., & Bouzon, Z. L. (2012). Alterations in architecture and metabolism induced by ultraviolet radiation-B in the carragenophyte Chondracanthus teedei (Rhodophyta, Gigartinales). Protoplasma, 249(2), 353–367. https://doi.org/10.1007/s00709-011-0286-1
Shao, S., Meyer, C. J., Ma, F., Peterson, C. A., & Bernards, M. A. (2007). The outermost cuticle of soybean seeds: Chemical composition and function during imbibition. Journal of Experimental Botany, 58(5), 1071–1082. https://doi.org/10.1093/jxb/erl268
Sidman, R. L., Motla, P. A., & Feder, N. (2001). Improved polyester wax embedding for histology. Biotechnic and Histochemistry, 36(5), 279–284. https://doi.org/10.3109/10520296109113291
Silué, S., Diarrassouba, N., Jesus Fofana, I., Muhovski, Y., Toussaint, A., Mergeai, G., Jacquemin, J. M., & Baudoin, J. P. (2013). Description of Phaseolus vulgaris L. aborting embryos from ethyl methanesulfonate (EMS) mutagenized plants. Biotechnology, Agronomy and Society and Environment, 17(4), 563–571. https://hdl.handle.net/2268/163623
Singh, H. B. (2016). Seed biopriming: A comprehensive approach towards agricultural sustainability. Indian Phytopathology, 69(3), 203–209.
Smith, C. M., Satoh, K., & Fork, D. C. (2008). The effects of osmotic tissue dehydration and air drying on morphology and energy transfer in two species of Porphyra. Plant Physiology, 80(4), 843–847. https://doi.org/10.1104/pp.80.4.843
Sreenivasulu, N., & Wobus, U. (2013). Seed-development programs: A systems biology-based comparison between dicots and monocots. Annual Review of Plant Biology, 64, 189–217. https://doi.org/10.1146/annurev-arplant-050312-120215
Terán, B. M. (2015). Evaluación de la eficiencia de la prueba del pH del exudado para estimar la viabilidad y vigor de las semillas de soya (Glycine max L.) [Tesis de Grado, Universidad Técnica Estatal de Quevedo]. Repositorio de la Universidad Técnica Estatal de Quevedo. https://repositorio.uteq.edu.ec/bitstream/43000/1938/1/T-UTEQ-0043.pdf
Tessmer, M. A., de Azevedo Kuhn, T. M., Appezzato-da-Glória, B., Lopes, J. R. S., Erler, G., & Bonani, J. P. (2022). Histology of damage caused by Euschistus heros (F.) nymphs in soybean pods and seeds. Neotropical Entomology, 51(1), 112–121. https://doi.org/10.1007/s13744-021-00931-w
ThermoFisher-Scientific. (2010). Safety data sheet of o-xylene. ThermoFisher-Scientific. https://bit.ly/3SyCHMh
Thorne, J. H. (2015). Chapter 1. Physiology of soybean seed development. In S. H. West (Ed.), Physiological-pathological interactions affecting seed deterioration (Vol. 12; pp. 1–10). Crop Science Society of America. https://doi.org/10.2135/cssaspecpub12.c1
Tnani, H. (2012). The structure and function of maize scutellum during early stages of germination [Tesis de Doctorado, Universidad de Barcelona] Repositorio de la Universidad de Barcelona. https://bit.ly/3bgPZNm
Waterworth, W. M., Bray, C. M., & West, C. E. (2015). The importance of safeguarding genome integrity in germination and seed longevity. Journal of Experimental Botany, 66(12), 3549–3558. https://doi.org/10.1093/jxb/erv080
Zhu, D., Larin, K. V., Luo, Q., & Tuchin, V. V. (2013). Recent progress in tissue optical clearing. Laser and Photonics Reviews, 7(5), 732–757. https://doi.org/10.1002/lpor.201200056
Zraidi, A., Pachner, M., Lelley, T., & Obermayer, R. (2003). On the genetics and histology of the hull-less character of styrian oil-pumpkin (Cucurbita pepo L.). Cucurbit Genetics Cooperative Report, 26, 57–61. https://cucurbit.info/wp-content/uploads/2019/03/cgc26-18.pdf
Additional Files
Published
How to Cite
Issue
Section
License
1. Proposed policy for open access journals
Authors who publish in this journal accept the following conditions:
a. Authors retain the copyright and assign to the journal the right to the first publication, with the work registered under the attribution, non-commercial and no-derivative license from Creative Commons, which allows third parties to use what has been published as long as they mention the authorship of the work and upon first publication in this journal, the work may not be used for commercial purposes and the publications may not be used to remix, transform or create another work.
b. Authors may enter into additional independent contractual arrangements for the non-exclusive distribution of the version of the article published in this journal (e.g., including it in an institutional repository or publishing it in a book) provided that they clearly indicate that the work was first published in this journal.
c. Authors are permitted and encouraged to publish their work on the Internet (e.g. on institutional or personal pages) before and during the review and publication process, as it may lead to productive exchanges and faster and wider dissemination of published work (see The Effect of Open Access).