Viabilidad de Ischaemum rugosum Salisb. determinada mediante la prueba de tetrazolio
DOI:
https://doi.org/10.15517/am.v32i1.40318Palabras clave:
malezas, temperatura de almacenamiento, imbibición, corte de semillasResumen
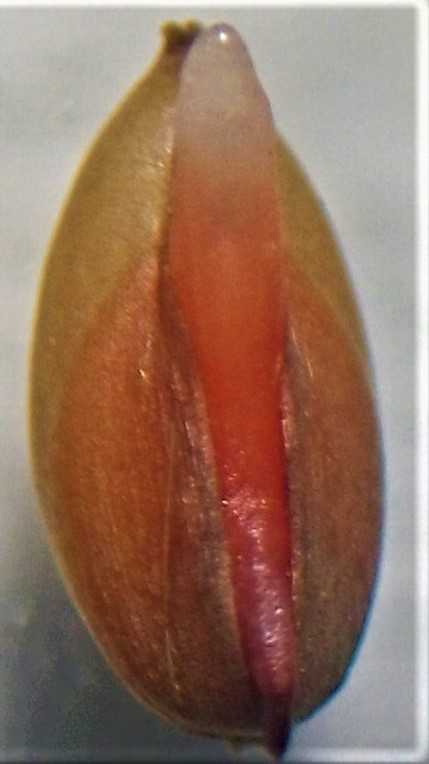
Introducción. La estimación de la viabilidad de Ischaemum rugosum Salisb. con la prueba de tetrazolio es una medida para el estudio de la dinámica biológica de esta maleza. Objetivo. Evaluar los factores que afectan la viabilidad de I. rugosum Salisb. mediante la prueba de tetrazolio. Materiales y métodos. El trabajo se realizó en el Laboratorio Oficial de Análisis de Calidad de Semillas del Centro para Investigaciones en Granos y Semillas de la Universidad de Costa Rica, San José, Costa Rica; en octubre de 2015. Las semillas de I. rugosum Salisb. se sometieron a los siguientes tratamientos previo a la prueba de tetrazolio: almacenamiento de semilla (temperatura ambiente: 23,9 °C y temperatura refrigerada: 5 °C), imbibición (embebida y no embebida), corte de la cariópside (con corte y sin corte) y unidad propagativa (cariópside pequeña, cariópside grande y espiguilla). Se contaron como viables las semillas teñidas de manera uniforme. Resultados. Hubo interacción significativa entre temperatura de almacenamiento y unidad propagativa, la cariópside grande tuvo una mayor ventaja con respecto a cariópside pequeña (12,18 a 1) o la espiguilla (181,27 a 1) y este resultado fue más contundente bajo temperatura ambiente. Hubo interacción significativa entre unidad propagativa y corte, todas las semillas requirieron de corte para que el tetrazolio lograra teñir los tejidos, fue más concluyente el efecto en la semilla grande que en la espiguilla (1326,1 a 1) y que en la pequeña (7,39 a 1). La interacción de las temperaturas de almacenamiento e imbibición y corte e imbibición fue significativa. Las semillas sometidas a temperatura ambiente, embebidas (41,26 a 1) y con corte e imbibición (18,17 a 1) fueron más viables. Conclusión. La imbibición y el corte facilitaron la tinción, la semilla grande y el almacenamiento en temperatura ambiente influyeron sobre la viabilidad de I. rugosum Salisb.
Descargas
Citas
Akanda, R. U., Mullahey, J. J., & Shilling, D. G. (1996). Environmental factors affecting germination of tropical soda apple (Solanum viarum). Weed Science, 44(3), 570–574. https://doi.org/10.1017/S0043174500094352
Alonso-Esquivel, M., Ortiz-López, Y., Ramos-Ramírez, R., Oliva-Diaz, H., & Capote-del Sol, M. (2011). Dormancia en semillas de papaya cv Maradol Roja durante el almacenamiento. Agronomía Mesoamericana, 22(2), 351–357. https://doi.org/10.15517/am.v22i2.11828
Aramendiz-Tatis, H., Cardona, C., Jarma, A., Robles, J., & Montalván, R. (2007). Efectos del almacenamiento en la calidad fisiológica de la semilla de berenjena (Solanum melongena L.). Agronomía Colombiana, 25(1), 104–112.
Awan, T. H., Chauhan, B. S., & Cruz, P. C. S. (2014). Physiological and morphological responses of Ischaemum rugosum Salisb. (Wrinkled Grass) to different nitrogen rates and rice seeding rates. PLoS One, 9(6), e98255. http://doi.org/10.1371/journal.pone.0098255
Benech-Arnold, R. L., Sánchez, R. A., Forcella, F., Kruk, B. C., & Ghersa, C. M. (2000). Environmental control of dormancy in weed seed banks in soil. Field crops research, 67(2), 105–122. https://doi.org/10.1016/S0378-4290(00)00087-3
Berjak, P & Pammenter, N. W. (2010). Manual de Semillas de Árboles Tropicales [Monografía]. Reforestation, Nurseries, & Genetics Resources. https://rngr.net/publications/manual-de-semillas-de-arboles-tropicales
Borza, J. K., Westerman, P. R., & Liebman, M. (2007). Comparing estimates of seed viability in three foxtail (Setaria) species using the imbibed seed crush test with and without additional tetrazolium testing. Weed Technology, 21(2), 518–522. https://doi.org/10.1614/WT-06-110
Doria, J. (2010). Generalidades sobre las semillas: su producción, conservación y almacenamiento. Cultivos Tropicales, 31(1), 74–75.
Giraldo-Cañas, D. (2010). Distribución e invasión de gramíneas C3 y C4 (Poaceae) en un gradiente altitudinal de los andes de Colombia. Caldasia, 32(1), 65–86.
Heap, I. (2020, July 1). The International Herbicide-Resistant Weed Database. WeedScience. http://www.weedscience.org/Home.aspx
International Rules for Seed Testing Association. (2014). Seed vigour testing. ISTA.
Hossain, M. A., Ishimine, Y., Akamine, H., Murayama, S., Uddin, S. M., & Kuniyoshi, K. (1999). Effect of burial depth on emergence of Panicum repens. Weed Science, 47(7), 651–656. https://doi.org/10.1017/S0043174500091281
Jarma-Orozco, A., Arvelaez, J., & Clavijo, J. (2007). Germinación de Ischaemum rugosum Salisb. en respuesta a estímulos ambientales y químicos. Temas Agrarios, 12(2), 31-41. https://doi.org/10.21897/rta.v12i2.1198
Jurado, E., & Westoby, M. (1992). Seedling growth in relation to seed size among species of arid Australia. Journal of Ecology, 80(3), 407–416. https://doi.org/10.2307/2260686
Leishman, M. R., Wright, I. J., Moles, A. T., & Westoby, M. (2000). The evolutionary ecology of seed size. En M. Fenner (Ed.), Seeds: the ecology of regeneration in plant communities (2a ed.) (pp. 31-57). Editorial. CAB International.
Marenco, R. A., & Santos, R. V. C. D. (1999). Wrinkledgrass and rice intra and interspecific competition. Revista Brasileira de Fisiologia Vegetal, 11(2), 107-111.
Ocampo-Sánchez, R. A. (1985). Incidencia de plantas indeseables en el cultivo de arroz (Oryza sativa) en el cantón de Aguirre y Parrita [Tesis de licenciatura no publicada]. Universidad de Costa Rica
Pabón, R. (1983). Algunos aspectos biológicos de la maleza falsa caminadora (Ischaemum rugosum) en los llanos orientales (Tesis de Maestría, Universidad Nacional de Colombia). Repositorio de la Corporación Colombiana de Investigación Agropecuaria. http://hdl.handle.net/20.500.12324/23911
Patil, V., & Dadlani, M. (2009). Handbook of seed testing. CABI Publishing.
Pérez, F. 2002. Material vegetal de reproducción: manejo conservación y tratamiento. Junta de Andalucía. http://www.juntadeandalucia.es/medioambiente/consolidado/publicacionesdigitales/80-402_MATERIAL_VEGETAL_DE_REPRODUCCION__MANEJO_CONSERVACION_Y_TRATAMIENTO/80-402/0_MATERIAL_VEGETAL_DE_REPRODUCCION.PDF
Rao, A. N., & Moody, K. (1995, July 24 to 28). Effect of seeding depth and soil moisture regime on emergence of Ischaemum rugosum and Echinochloa glabrescens. ResearchGate. https://www.researchgate.net/publication/286623927_Effect_of_seeding_depth_and_soil_moisture_regime_on_emergence_of_Ischaemum_rugosum_and_Echinochloa_glabrescens
Rao, N. K., Hanson, J., Dulloo, M. E., & Ghosh, K. (2007). Manual para el Manejo de Semillas en Bancos de Germoplasma (Manuales para Bancos de Germoplasma No. 8). Bioversity Intenational. https://www.bioversityinternational.org/fileadmin/_migrated/uploads/tx_news/Manual_para_el_manejo_de_semillas_en_bancos_de_germoplasma_1261_01.pdf
Rodríguez Quilón, I., Adam, G., & Durán, J. M. (2008). Ensayos de germinación y análisis de viabilidad y vigor en semillas. Agricultura: Revista Agropecuaria y Ganadera, 78(912), 836–842.
Vázquez-Yanes, C., & Cervantes, V. (1997). La reproducción de las plantas, semillas y meristemos. Biblioteca Virtual del Instituto Latinoamericano de la Comunicación Educativa. http://bibliotecadigital.ilce.edu.mx/sites/ciencia/volumen3/ciencia3/157/htm/lcpt157.htm
Westoby, M., Jurado, E., & Leishman, M. (1992). Comparative evolutionary ecology of seed size. Trends in Ecology & Evolution, 7(11), 368–372. https://doi.org/10.1016/0169-5347(92)90006-W
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
1. Política propuesta para revistas de acceso abierto
Los autores/as que publiquen en esta revista aceptan las siguientes condiciones:
- Los autores/as conservan los derechos morales de autor y ceden a la revista el derecho de la primera publicación, con el trabajo registrado con la licencia de atribución, no comercial y sin obra derivada de Creative Commons, que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista, no se puede hacer uso de la obra con propósitos comerciales y no se puede utilizar las publicaciones para remezclar, transformar o crear otra obra.
- Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en esta revista (p. ej., incluirlo en un repositorio institucional o publicarlo en un libro) siempre que indiquen claramente que el trabajo se publicó por primera vez en esta revista.
- Se permite y recomienda a los autores/as a publicar su trabajo en Internet (por ejemplo en páginas institucionales o personales) antes y durante el proceso de revisión y publicación, ya que puede conducir a intercambios productivos y a una mayor y más rápida difusión del trabajo publicado (vea The Effect of Open Access).