Abstract
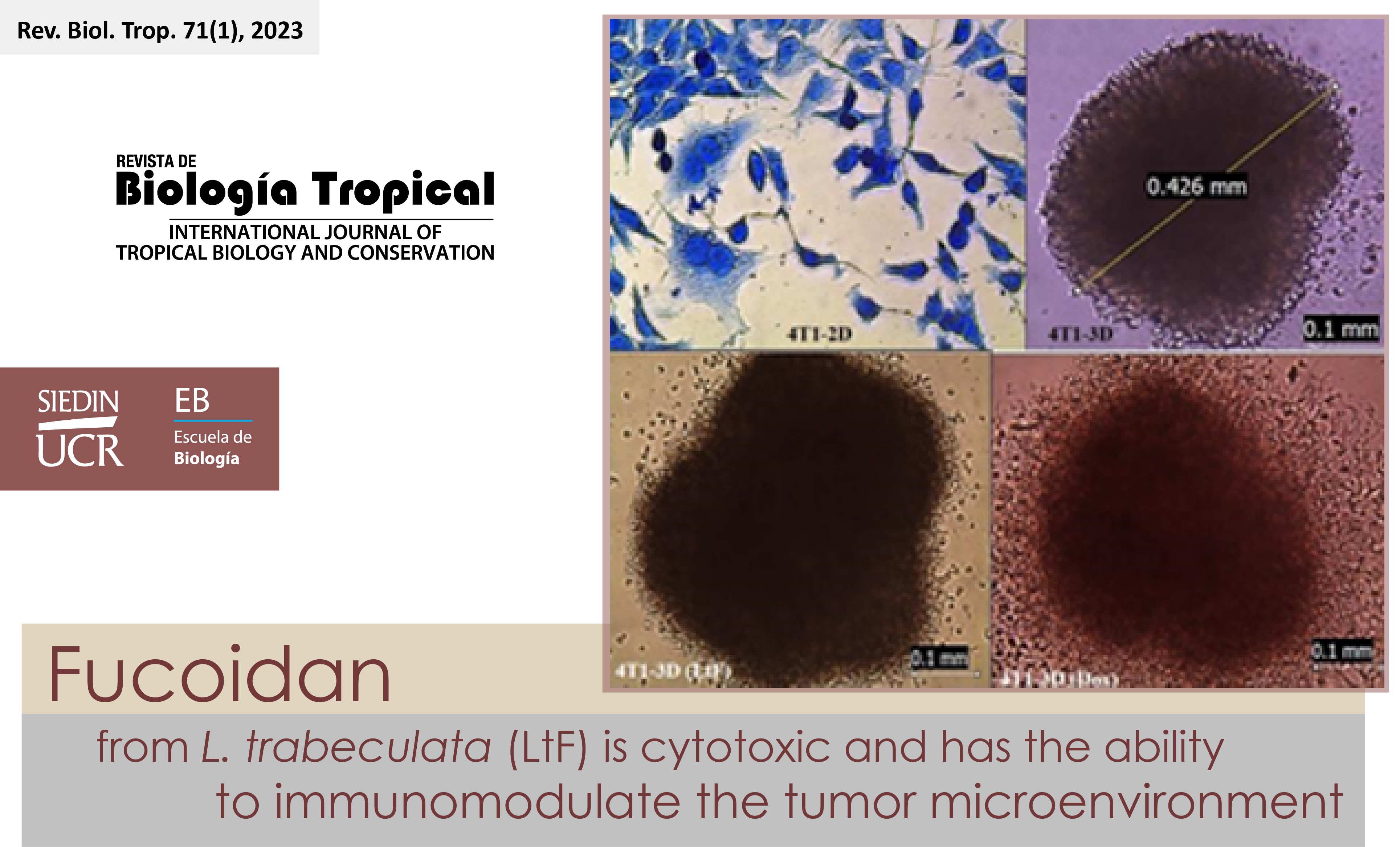
Introduction: The therapeutic benefits of the brown algae fucoidan in the treatment of breast cancer have attracted considerable interest in recent years. However, research using spheroids which provide relevant results in trials for antitumor and immunomodulatory products because they adequately simulate the tumor microenvironment, is limited. Objective: To evaluate the antitumor and immunomodulatory activity of Lessonia trabeculata fucoidan (LtF), native to the Peruvian Sea, on two types of multicellular tumor spheroids. Methods: The study was conducted from January to December 2021. Two types of spheroides were elaborated: from 4T1 tumor cells (MTS), and from 4T1 tumor cells+mouse splenocytes (MTSs). The antitumor activity of LtF was evaluated in MTS by quantifying cell viability with MTT. Immunomodulatory activity was determined in MTSs using the IC50 for two types of treatment: simple, fucoidan alone (LtF) and combined, fucoidan+doxorubicin (LtF+Dox). Pro-inflammatory (TNF-α, IL-6) and anti-inflammatory (IL-10, TGF-β) cytokine production was quantified by sandwich ELISA 72 h after treatment. Dox was used as positive control in all assays. Results: LtF exerted antitumor activity as evidenced by increased necrotic zone and cell debris formation compared to the untreated control. Antitumor activity was concentration dependent between 100 and 6 000 μg/ml. In MTSs, simple treatment increased IL-6 and decreased IL-10 and TGF-β production. The combined treatment significantly reduced TGF-β production. In both treatments and Dox, there was an increase in IL-6 compared to the untreated control. The highest production of IL-10 and TGF-β was observed in the untreated control, compatible with a highly immunosuppressive tumor microenvironment. Conclusions: LtF is a good candidate for the treatment of breast cancer and can immunomodulate the tumor microenvironment alone or in combination with Dox.
References
Abudabbus, A., Badmus, J. A., Shalaweh, S., Bauer R., & Hiss, D. (2017). Effects of fucoidan and chemotherapeutic agent combinations on malignant and non-malignant breast cell lines. Current Pharmaceutical Biotechnology, 18(9), 748-757.
Atashrazm, F., Lowenthal, R. M., Woods, G. M., Holloway, A. F., & Dickinson, J. L. (2015). Fucoidan and cancer: a multifunctional molecule with anti-tumor potential. Marine Drugs, 13(4), 2327-2346.
Berraondo, P., Sanmamed, M. F., Ochoa, M. C., Etxeberria, I., Aznar, M. A., Pérez-Gracia, J. L., Rodríguez-Ruiz, M. E., Ponz-Sarvise, M., Castañón, E., & Melero I. (2019). Cytokines in clinical cancer immunotherapy. British Journal of Cancer, 120(1), 6-15.
Boschi, C., Maldonado, H., Ly, M., & Guibal, E. (2011). Cd(II) biosorption using Lessonia kelps. Journal of Colloid and Interface Science, 357(2), 487-496.
Bożyk, A., Wojas-Krawczyk, K., Krawczyk, P., & Milanowski J. (2022). Tumor microenvironment- a short review of cellular and interaction diversity. Biology (Basel), 11(6):929.
Campos, L., Berrios, F., Oses, R., González, J. E., & Bonnail, E. (2021). Unravelling Lessonia trabeculata management in coastal areas of the Atacama region of northern Chile through a DPSIR approach: Insights for sustainable plans. Marine Policy, 133, 104737.
Chen, J., Wei, Y., Yang, W., Huang, Q., Chen, Y., Zeng, K., & Chen, J. (2022). IL-6: The link between inflammation, immunity and breast cancer. Frontiers in Oncology, 18;12, 903800.
Chen, L. M., Yang, P. P., Al Haq, A., Hwang, P. A., Lai, Y. C., Weng, Y. S., Chen, M., & Hsu, H. L. (2022a). Oligo-Fucoidan supplementation enhances the effect of Olaparib on preventing metastasis and recurrence of triple-negative breast cancer in mice. Journal of Biomedical Science, 29, 70.
Colona, E., Alzamora, L., Chávez, J., Apumayta, E., & Chang, I. (2019). Incremento de la viabilidad, producción de especies reactivas de oxígeno, IL-1 y TNF-α en células mononucleares de sangre periférica humana tratadas con fucoidan de Lessonia trabeculata. Revista Peruana de Biología, 26(3), 291-300.
Colona Vallejos, E. (2022). Estudio de las propiedades inmunomoduladora y antitumoral del fucoidan de Lessonia trabeculata (Villouta & Santelices, 1986) [Doctor dissertation, Universidad Nacional Mayor de San Marcos, Lima, Perú]. Cybertesis UNMSM. https://hdl.handle.net/20.500.12672/18372.
Gallardo, J. C., Espinosa, M., Meléndez, J. & Maldonado, V. (2006). Esferoides tumorales multicelulares en la evaluación de estrategias terapéuticas anticancerosas. Revista de Educación Bioquímica, 25(004), 101-107.
Giaquinto, A. N., Sung, H., Miller, K. D., Kramer, J. L., Newman, L. A., Minihan, A., Jemal, A., & Siegel, R. L. (2022). Breast Cancer Statistics, CA: A Cancer Journal for Clinicians, 72(6): 524-541.
González, P., Edding, M., Torres, R., & Manríquez P. H. (2018). Increased temperature but not pCO2 levels affect early developmental and reproductive traits of the economically important habitat-forming kelp Lessonia trabeculata. Marine Pollution Bulletin, 135: 694-703.
Gouraguine, A.. Moore, P., Burrows, M. T., Velasco, E., Ariz, L., Figueroa-Fábrega, L., Muñoz-Cordovez, R., Fernandez-Cisternas, I., Smale, D., & Perez-Matus, A. (2021). The intensity of kelp harvesting shapes the population structure of the foundation species Lessonia trabeculata along the chilean coastline. Marine Biology, 168, 66.
Guo, R., Deng, M., He, X., Li, M., Li, J., He, P., Liu, H., Li, M., Zhang, Z., & He, Q. (2022). Fucoidan functionalized activated platelet-hitchhiking micelles simultaneously track tumor cells and remodel the immunosuppressive microenvironment for efficient metastatic cancer treatment. Acta Pharmaceutica Sinica B, 12(1), 467-482.
Hsieh, C. C., & Wang, C. H. (2018). Aspirin disrupts the crosstalk of angiogenic and inflammatory cytokines between 4T1 breast cancer cells and macrophages. Mediators of Inflammation, 2018, 6380643.
Hsu, H.-Y., & Hwang, P. A. (2019). Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clinical and Translational Medicine, 8(1), 15.
Hsu, H.-Y., Lin, T.-Y., Hwang, P.-A., Tseng, L.-M., Chen, R.-H., Tsao, S.-M., & Hsu, J. (2013). Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGF receptor degradation in breast cancer. Carcinogenesis, 34(4), 874-884.
Jin, J.O., Chauhan, P. S., Arukha, A. P., Chavda, V., Dubey, A., & Yadav, D. (2021). The therapeutic potential of the anticancer activity of fucoidan: Current advances and hurdles. Marine Drugs, 19(5), 265.
Kiselevskiy, M. V., Anisimova, N. Y., Ustyuzhanina, N. E., Vinnitskiy, D. Z., Tokatly, A. I., Reshetnikova, V. V., Chikileva, I. O., Shubina, I. Z., Kirgizov, K. I., & Nifantiev, N. E. (2022). Perspectives for the use of fucoidans in clinical oncology. International Journal of Molecular Sciences, 23(19), 11821.
Lainetti, P. F., Leis-Filho A. F., Laufer-Amorim, R., Battazza, A., & Fonseca-Alves, C. E. (2020). Mechanisms of resistance to chemotherapy in breast cancer and possible targets in drug delivery systems. Pharmaceutics, 12(12), 1193.
Lan, T., Chen, L., & Wei, X. (2021). Inflammatory cytokines in cancer: Comprehensive understanding and clinical progress in gene therapy. Cells, 10(1),100.
Lee, H. M., Lee, H. J., & Chang, J. E. (2022). Inflammatory cytokine: An attractive target for cancer treatment. Biomedicines, 10(9), 2116.
Lee, H.-G., L., Nagahawatta, D. P., Liyanage, N. M., Jayawardhana, H. H. A. C. K., Yang, F., Je, J.-G., Kang, M.-C., Kim, H.-S., Jeon, & Y.-J., Jeon. (2022). Structural characterization and anti-inflammatory activity of fucoidan isolated from Ecklonia maxima stipe. Algae, 37(3), 239-247.
Li, Y., Gao, P., Yang, J., Yu, H., Zhu, Y., & Si, W. (2014). Relationship between IL-10 expression and prognosis in patients with primary breast cancer. Tumour Biology, 35(11), 11533-11540.
Lin, Y., Qi, X., Liu, H., Xue, K., Xu, S., & Tian, Z. (2020). The anti-cancer effects of fucoidan: a review of both in vivo and in vitro investigations. Cancer Cell International, 20, 154.
Ma, W.-P., Li, H.-H., Liu, M., & Liu, H.-B. (2021). Effects of simulated digestion in vitro on the structure and macrophages activation of fucoidan from Sargassum fusiforme. Carbohydrate Polymers, 272, 118484.
Malyarenko, O. S., Malyarenko, T. V., Usoltseva, R. V., Silchenko, A. S., Kicha, A. A., Ivanchina, N. V., & Ermakova, S. P. (2021). Fucoidan from brown algae Fucus evanescens potentiates the anti-proliferative efficacy of asterosaponins from starfish Asteropsis carinifera in 2D and 3D models of melanoma cells. International Journal of Biological Macromolecules, 185(6), 31–39.
Mayer, A. M. S., Guerrero, A. J., Rodríguez, A. D., TaglialatelaScafati, O., Nakamura, F., & Fusetani, N. (2019). Marine pharmacology in 2014–2015: marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities: affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Marine Drugs, 18(1), 5.
Nii, T., Makino, K., & Tabata, Y. (2020). Three-Dimensional Culture System for Drug Screening. Cancers, 12(10), 2754.
Nilofar Danishmalik, S., Lee, S. H., & Sin, J. I. (2017). Tumor regression is mediated via the induction of HER263-71- specific CD8+ CTL activity in a 4T1.2/HER2 tumor model: no involvement of CD80 in tumor control. Oncotarget, 8(16), 26771-26788.
Park, H. B., Hwang, J., Lim, S. M., Zhang, W., & Jin, J. O. (2020). Dendritic cell-mediated cancer immunotherapy with Ecklonia cava fucoidan. International Journal of Biological Macromolecules, 159, 941-947.
Paulsen, Ø., Laird, B., Aass, N., Lea, T., Fayers, P., Kaasa, S., & Klepstad, P. (2017). The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLoS One, 12(5), e0177620.
Santelices, B., Castilla, J.C., Cancino, J., & Schmiede, P. (1980). Comparative ecology of Lessonia nigrescens and Durvillaea antarctica (Phaeophyta) in Central Chile. Marine Biology, 59, 119-132.
Syukri, A., Budu, H. M., Amir, M., Rohman, M. S., Mappangara, I., Kaelan, C., Wahyuni, S., Bukhari, A., Junita, A. R., Primaguna, M. R., Dwiyanti, R., & Febrianti, A. (2022). Doxorubicin induced immune abnormalities and inflammatory responses via HMGB1, HIF1-α and VEGF pathway in progressive of cardiovascular damage. Annals of Medicine and Surgery, 76, 103501.
Takahashi, H., Kawaguchi, M., Kitamura, K., Narumiya, S., Kawamura, M., Tengan, I., Nishimoto, S., Hanamure, Y., Majima, Y., Tsubura, S., Teruya, K., & Shirahata, S. (2018). An exploratory study on the anti-inflammatory effects of fucoidan in relation to quality of life in advanced cancer patients. Integrative Cancer Therapies, 17(2), 282-291.
Tala, F., Edding, M., & Vasquez, J. A. (2004). Aspects of the reproductive phenology of Lessonia trabeculata (Laminariales: Phaeophyceae) from three populations in northern Chile. New Zealand Journal of Marine and Freshwater Research, 38(2), 255-266.
Tevis, K. M., Colson, Y. L., & Grinstaff, M. W. (2017). Embedded spheroids as models of the cancer microenvironment. Advanced Biosystems, 1(10), 1700083.
Xue, M., Ge, Y., Zhang, J., Wang, Q., Hou, L., Liu, Y., Sun, L., & Li, Q. (2012). Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS One, 7(8), e43483.
Xue, M., Ge, Y., Zhang, J., Liu, Y., Wang, Q., Hou, L., & Zheng, Z. (2013). Fucoidan inhibited 4T1 mouse breast cancer cell growth in vivo and in vitro via downregulation of Wnt/β-catenin signaling. Nutrition and Cancer, 65(3), 460-468.
Xue, M., Liang, H., Tang, Q., Xue, C., HE, X.; Zhang, L., Zhang, Z., Liang, Z., Bian, K., Zhang, L., & Li, Z. (2017). The protective and immunomodulatory effects of fucoidan against 7, 12-Dimethyl benz[a]anthracene-induced experimental mammary carcinogenesis through the PD1/PDL1 signaling pathway in rats. Nutrition and Cancer, 69(8), 1234-1244.
Wang, S.-H, Huang, C.-Y., Chen, C.-Y., Chang, C.-C., Huang, C.-Y., Dong, C.-D., & Chang, J.-S. (2020). Structure and biological activity analysis of fucoidan isolated from Sargassum siliquosum. ACS Omega, 5(50), 32447-32455.
Zhang, W., An, E. K., Park, H. B., Hwang, J., Dhananjay, Y., Kim, S. J., Eom, H.Y., Oda, T., Kwak, M., Lee, P., & Jin, J. O. (2021). Ecklonia cava fucoidan has potential to stimulate natural killer cells in vivo. International Journal of Biological Macromolecules, 185, 111-121.
##plugins.facebook.comentarios##

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Revista de Biología Tropical