Abstract
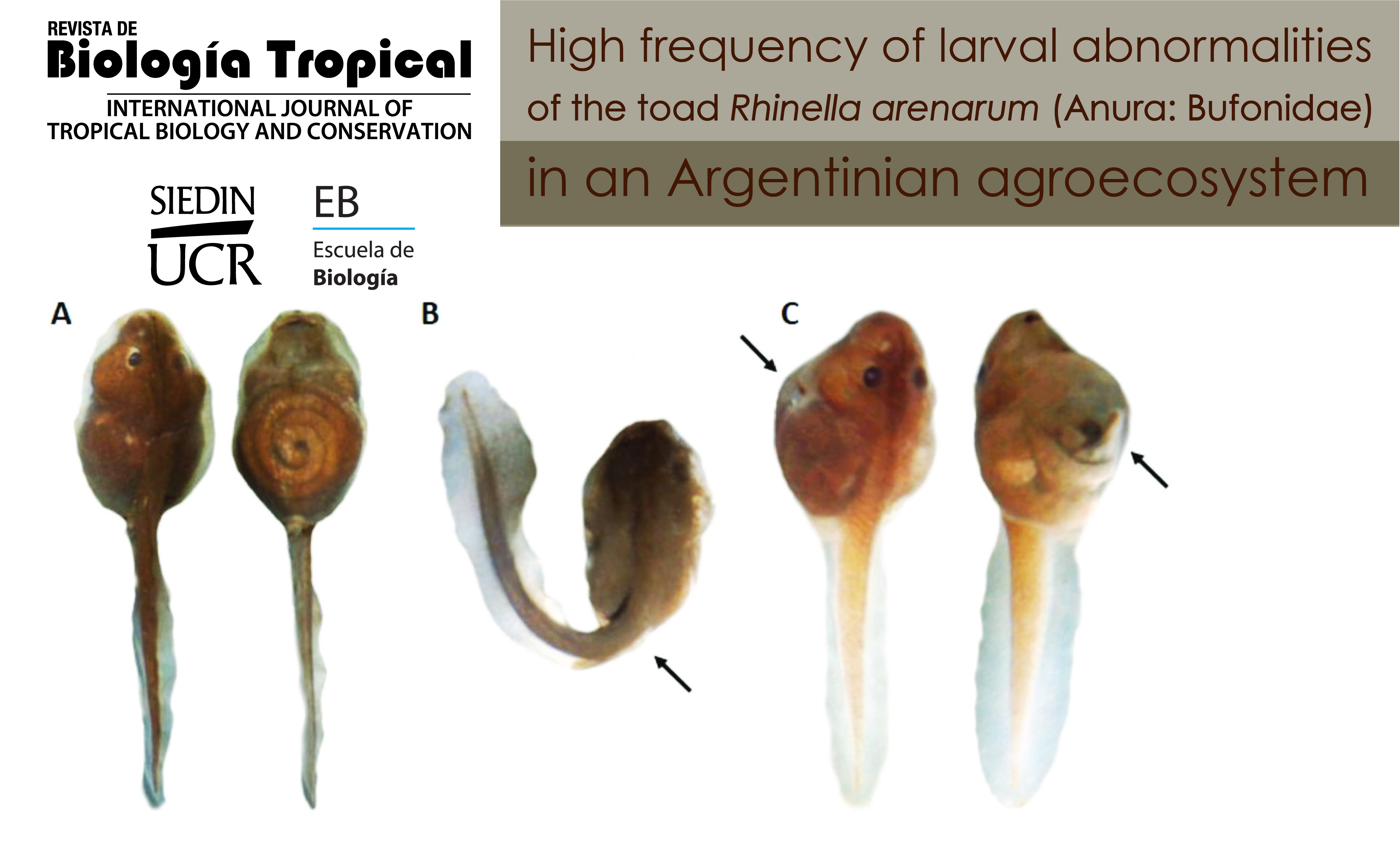
Introduction: The frequent use of pesticides is currently considered a cause of environmental pollution due to the high rate of entry of these substances into agroecosystems. This constitutes a risk for the species that inhabit these ecosystems, in particular anurans whose characteristics make them prone to exposure to and interaction with environmental pollutants. Objective: To report the occurrence of abnormalities in larvae of the common toad Rhinella arenarum inhabiting ponds surrounded by agroecosystems. Methods: In two consecutive springs (2015 and 2016), reproductive events of common toads were monitored in temporary pond systems in agricultural and non-agricultural areas, located near the city of La Plata (Buenos Aires, Argentina). The physicochemical parameters of the ponds were measured, and the stage of each reproductive event was recorded, such as the numbers of adult toads, amplexus and clutches. In the laboratory, the larvae were measured and photographed, their stage of development was recorded, and their morphology was examined under a stereomicroscope. Representative samples (normal and abnormal) from each pond studied were processed for histopathological analysis. Results: In the field studies carried out on a population of R. arenarum collected in an agroecosystem, a lower number of reproductive adults and clutches were observed in relation to the population of a non-agricultural pond. A total of 1 910 larvae were collected: 529 and 1 381 larvae from ponds located in non-agricultural and agricultural areas, respectively. Larvae from the agroecosystem showed two types of abnormalities: severe tail flexure and abdominal bloating. In addition, five degrees of severity could be determined in relation to abdominal bloating. Conclusions: This work reports the high frequency and severity of abnormalities observed in the early stages of R. arenarum larvae living within an agroecosystem, providing evidence of the negative impact that agricultural activities cause on aquatic ecosystems surrounded by farming areas.
References
Agostini, M. G., Kacoliris, F., Demetrio, P., Natale, G. S., Bonetto, C., & Ronco, A. E. (2013). Abnormalities in amphibian populations inhabiting agroecosystems in northeastern Buenos Aires Province, Argentina. Diseases of Aquatic Organisms, 104(2), 163–171.
Agostini, M. G., Natale, G. S., & Ronco, A. (2010). Lethal and sublethal effects of cypermethrin to Hypsiboas pulchellus tadpoles. Ecotoxicology, 19(8), 1545–1550.
Anzaldua, S. P., & Goldberg, J. (2019). Hotspot of tadpole abnormality in suburban south-west Florida. Herpetological Journal, 29(2), 115–124.
Aronzon, C. M., Sandoval, M. T., Herkovits, J., & Pérez-Coll, C. S. (2011). Stage-dependent toxicity of 2,4-dichlorophenoxyacetic on the embryonic development of a South American toad, Rhinella arenarum. Environmental Toxicology, 26(4), 373–381.
Baird, D. J., Brown, S. S., Lagadic, L., Liess, M., Maltby, L., Moreira-Santos, M., & Scott, G. I. (2007). In situ-based effects measures: Determining the ecological relevance of measured responses. Environmental Assessment and Management: An International Journal, 3(2), 259–267.
Bantle, J. A. (1991). Atlas of Abnormalities: A Guide for the Performance of FETAX. Oklahoma State University, Printing Services.
Beebee, T. J. & Griffiths, R. A. (2005). The amphibian decline crisis: a watershed for conservation biology? Biological Conservation, 125(3), 271–285.
Bindraban, P. S., Bulte, E. H., & Conijn, S. G. (2009). Can large-scale biofuels production be sustainable by 2020? Agricultural Systems, 101(3), 197–199.
Blaustein, A. R., & Wake, D. B. (1995). The puzzle of declining amphibian populations. Scientific American, 272(4), 52–57.
Boccioni, A. P. C., Lajmanovich, R. C., Peltzer, P. M., Attademo, A. M., & Martinuzzi, C. S. (2020). Toxicity assessment at different experimental scenarios with glyphosate, chlorpyrifos and antibiotics in Rhinella arenarum (Anura: Bufonidae) tadpoles. Chemosphere, 273, 128475.
Brunelli, E., Bernabò, I., Berg, C., Lundstedt-Enkel, K., Bonacci, A., & Tripepi, S. (2009). Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behaviour in Bufo bufo tadpoles. Aquatic Toxicology, 91(2), 135–142.
Brodeur, J. C., Suarez, R. P., Natale, G. S., Ronco, A. E., & Zaccagnini, M. E. (2011). Reduced body condition and enzymatic alterations in frogs inhabiting intensive crop production areas. Ecotoxicology and Environmental Safety, 74(5), 1370–1380.
Brodeur, J. C., & Vera-Candioti, J. (2017). Impacts of agricult ure and pesticides on amphibian terrestrial life stages: Potential biomonitor/bioindicator species for the Pampa region of Argentina. In M. L. Larramendy (Ed.), Ecotoxicology and Genotoxicology: Non-traditional Terrestrial Models Issues in Toxicology No. 32. (pp. 163–194). The Royal Society of Chemistry.
Camilión, M. C., Manassero, M. J., Hurtado, M. A., & Ronco, A. E. (2003). Copper, lead and zinc distribution in soils and sediments of the south-western coast of the Río de la Plata estuary. Journal of Soils and Sediments, 3(3), 213–220.
Carvalho, F. P. (2017). Mining industry and sustainable development: time for change. Food and Energy Security, 6(2), 61–77.
Collins, J. P., Crump, M. L., & Lovejoy III, T. E. (2009). Extinction in our Times: Global Amphibian Decline. Oxford University Press.
Croteau, M. C., Davidson, M. A., Lean, D., & Trudeau, V. (2008). Global increases in ultraviolet B radiation: potential impacts on amphibian development and metamorphosis. Physiological and Biochemical Zoology, 81(6), 743–761.
Davidson, C., Shaffer, H. B., & Jennings, M. R. (2002). Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conservation Biology, 16(6), 1588–1601.
Demetrio, P. M. (2012). Estudio de efectos biológicos de plaguicidas utilizados en cultivos de soja RR y evaluación de impactos adversos en ambientes acuáticos de agroecosistemas de la región pampeana. Universidad Nacional de La Plata.
Denoël, M., D’Hooghe, B., Ficetola, G. F., Brasseur, C., De Pauw, E., Thomé, J. P., & Kestemont, P. (2012). Using sets of behavioral biomarkers to assess short-term effects of pesticide: a study case with endosulfan on frog tadpoles. Ecotoxicology, 21(4), 1240–1250.
Evenson, R. E., & Gollin, D. (2003). Assessing the impact of the Green Revolution, 1960 to 2000'. Science, 300(5620), 758–762.
Gosner, K. L. (1960). A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica, 16(3), 183–190.
Guzy, J. C., McCoy, E. D., Deyle, A. C., Gonzalez, S. M., Halstead, N., & Mushinsky, H. R. (2012). Urbanization interferes with the use of amphibians as indicators of ecological integrity of wetlands. Journal of Applied Ecology, 49(4), 941–952.
Haywood, L. K., Alexander, G. J., Byrne, M. J., & Cukrowska, E. (2004). Xenopus laevis embryos and tadpoles as models for testing for pollution by zinc, copper, lead and cadmium. African Zoology, 39(2), 163–174.
Henle, K., & Dubois, A. (2017). Studies on anomalies in natural populations of amphibians. Mertensiella, 25, 185–242.
Heyer, W. R. (1994). Variation within the Leptodactylus podicipinus-wagneri complex of frogs (Amphibia: Leptodactylidae). Smithsonian Contributions to Zoology, 446, 1–132.
Hopkins, W. A., Congdon, J., & Ray, J. K. (2000). Incidence and impact of axial malformations in larval bullfrogs (Rana catesbeiana) developing in sites polluted by a coal-burning power plant. Environmental Toxicology and Chemistry: An International Journal, 19(4), 862–868.
Houlahan, J. E., Findlay, C. S., Schmidt, B. R., Meyer, A. H., & Kuzmin, S. L. (2000). Quantitative evidence for global amphibian population declines. Nature, 404(6779), 752.
Johnson, P. T., Lunde, K. B., Ritchie, E. G., Reaser, J. K., & Launer, A. E. (2001). Morphological abnormality patterns in a California amphibian community. Herpetologica, 57(3), 336–352.
Lajmanovich, R. C., Attademo, A. M., Peltzer, P. M., Junges, C. M., & Cabagna, M. C. (2011). Toxicity of four herbicide formulations with glyphosate on Rhinella arenarum (Anura: Bufonidae) tadpoles: B-esterases and glutathione S-transferase inhibitors. Archives of Environmental Contamination and Toxicology, 60(4), 681–689.
Lajmanovich, R. C., Peltzer, P. M., Attademo, A. M., Cabagna-Zenklusen, M. C., & Junges, C. M. (2012). Los agroquímicos y su impacto en los anfibios: un dilema de difícil solución. Química Viva, 11(3), 184–198.
Lannoo, M. J. (2008). Malformed frogs: The Collapse of Aquatic Ecosystems. University of California Press.
Lunde, K. B., & Johnson, P. T. (2012). A practical guide for the study of malformed amphibians and their causes. Journal of Herpetology, 46(4), 429–442.
Mac Loughlin, T. M., Peluso, M. L., & Marino, D. J. G. (2017). Pesticide impact study in the peri-urban horticultural area of Gran La Plata, Argentina. Science of the Total Environment, 598, 572–580.
Natale, G. S. (2006). Análisis ecotoxicológico de una comunidad de anuros de la Región Pampeana. Facultad de Ciencias Naturales y Museo.
Natale, G. S., Vera-Candioti, J., Ruiz de Arcaute, C., Soloneski, S., Larramendy, M. L., & Ronco, A. E. (2018). Lethal and sublethal effects of the pirimicarb-based formulation Aficida® on Boana pulchella (Duméril and Bibron, 1841) tadpoles (Anura, Hylidae). Ecotoxicology and Environmental Safety, 147, 471–479.
Peluso, M. L. (2011). Evaluación de efectos biológicos y biodisponibilidad de contaminantes en sedimentos del Río de la Plata y afluentes. Facultad de Ciencias Exactas.
Peluso, M. L., Giusto, A., Rossini, G. D. B., Ferrari, L., Salibián, A., & Ronco, A. E. (2011). Hyalella curvispina (amphipoda) as a test organism in laboratory toxicity testing of environmental samples. Fresenius Environmental Bulletin, 20(2), 372–376.
Pérez-Coll, C. S., Herkovits, J., & Salibián, A. (1988). Embryotoxicity of lead on Bufo arenarum. Bulletin of Environmental Contamination and Toxicology, 41(2), 247–252.
Pérez-Iglesias, J., Soloneski, S., Nikoloff, N., Natale, G. S., & Larramendy, M. (2015). Toxic and genotoxic effects of the imazethapyr-based herbicide formulation Pivot H® on montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicology and Environmental Safety, 119, 15–24.
Peltzer, P. M., Lajmanovich, R. C., Sanchez, L. C., Attademo, A. M., Junges, C. M., Bionda, C. L., Martino, A., & Basso, A. (2011). Morphological abnormalities in amphibian populations. Herpetological Conservation and Biology, 6(3), 432–442.
Plaul, S. E., Andrés-Laube, P. F., Pacheco Marino, S. G., Santamaría Martín, C. J., Moyano, D. A., & Barbeito, C. G. (2017). Morphological techniques used in ichthyopathological diagnosis. In A. Méndez-Vilas (Ed.), Microscopy and imaging science approaches to applied research and education (pp. 269–280). Badajoz.
Primost, J. E., Marino, D. J., Aparicio, V. C., Costa, J. L., & Carriquiriborde, P. (2017). Glyphosate and AMPA,“pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem, Argentina. Environmental Pollution, 229, 771–779.
Quirós, R., Rosso, J. J., Rennella, A., Sosnovsky, A., & Boveri, M. (2002). Análisis del estado trófico de las lagunas pampeanas (Argentina). Interciencia, 27(11), 584–591.
R Development Core Team. (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available in: http://www.R-project.org/
Rasband, W. (1997). ImageJ. US National Institutes of Health, Bethesda, MD, USA.
Read, J. (1997). Comparative abnormality rates of the trilling frog at Olympic Dam. Herpetofauna-Sydney, 27, 23–27.
Reeves, M. K., Dolph, C. L., Zimmer, H., Tjeerdema, R. S., & Trust, K. A. (2008). Road proximity increases risk of skeletal abnormalities in wood frogs from National Wildlife Refuges in Alaska. Environmental Health Perspectives, 116(8), 1009–1014.
Ruiz, A. M., Maerz, J. C., Davis, A. K., Keel, M. K., Ferreira, A. R., Conroy, M. J., Morris, L. A., & Fisk, A. T. (2010). Patterns of development and abnormalities among tadpoles in a constructed wetland receiving treated wastewater. Environmental Science & Technology, 44(13), 4862–4868.
Sansiñena, J. A., Peluso, M. L., Costa, C. S., Demetrio, P. M., Mac Loughlin, T. M., Marino, D. J., Alcalde, L., & Natale, G. S. (2018). Evaluation of the toxicity of the sediments from an agroecosystem to two native species, Hyalella curvispina (Crustacea: Amphipoda) and Boana pulchella (Amphibia: Anura), as potential environmental indicators. Ecological Indicators, 93, 100–110.
Severtsova, E. A., Aguillon-Gutierrez, D. R., & Severtsov, A. S. (2012). Frequent anomalies in larvae of common and moor frogs in Moscow area and in the Suburbs of Moscow, Russia. Russian Journal of Herpetology, 19(4), 337–348.
Silva, N., & Toledo, L. (2010). Bokermannohyla saxicola (NCN), Scinax curicica (Lanceback Treefrog), Scinax squalirostris (Snouted Treefrog), Trachycephalus mesophaeus (Golden-eyed Treefrog), and Elachistocleis sp. (Oval Frog). Morphology Herpetological Review, 41(3), 333–334.
Sinsch, U. (1990). Migration and orientation in anuran amphibians. Ethology Ecology & Evolution, 2(1), 65–79.
Sparling, D. W., Linder, G., Bishop, C. A., & Krest, S. (2010). Ecotoxicology of Amphibians and Reptiles. CRC Press.
Svartz, G., Aronzon, C., & Coll, C. P. (2016). Comparative sensitivity among early life stages of the South American toad to cypermethrin-based pesticide. Environmental Science and Pollution Research, 23(3), 2906–2913.
Venturino, A., Rosenbaum, E., Caballero de Castro, A., Anguiano, O. L., Gauna, L., Fonovich de Schroeder, T., & Pechen de D'Angelo, A. M. (2003). Biomarkers of effect in toads and frogs. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals, 8(3–4), 167–186.
Wilbur, H. M., & Semlitsch, R. D. (1990). Ecological consequences of tail injury in Rana tadpoles. Copeia, 1, 18–24.
Young, B. E., Stuart, S. N., Chanson, J. S., Cox, N. A., & Boucher, T. M. (2004). Joyas que están desapareciendo: El estado de los anfibios en el Nuevo Mundo. NatureServe.
Zar, J. H. (2013). Biostatistical analysis. Pearson.
##plugins.facebook.comentarios##

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Revista de Biología Tropical